What is Cathodic Protection? Types of cathodic protection
Introduction
Corrosion is a perennial problem for metals exposed to corroding elements such as seawater and chemicals. In shipping, if corrosion is allowed unabated development, it can lead to a quick erosion of metals in critical locations.
As a result, staying on top of corrosion problems from the beginning is very important. Cathodic protection is one of many ways to ensure the corrosion-free operation of vulnerable structures such as foundations, pipelines, and process equipment.
In this article, we explore what cathodic protection is, how it works and the different types of cathodic protection methods in use. Let’s begin.
What is cathodic protection?
Cathodic protection is the manipulation of electrochemical reactions between metals to divert the corrosion process from important components to non-critical components.
When applied correctly, cathodic protection can completely eliminate corrosion and give a long life to protected parts.
Why do we need cathodic protection?
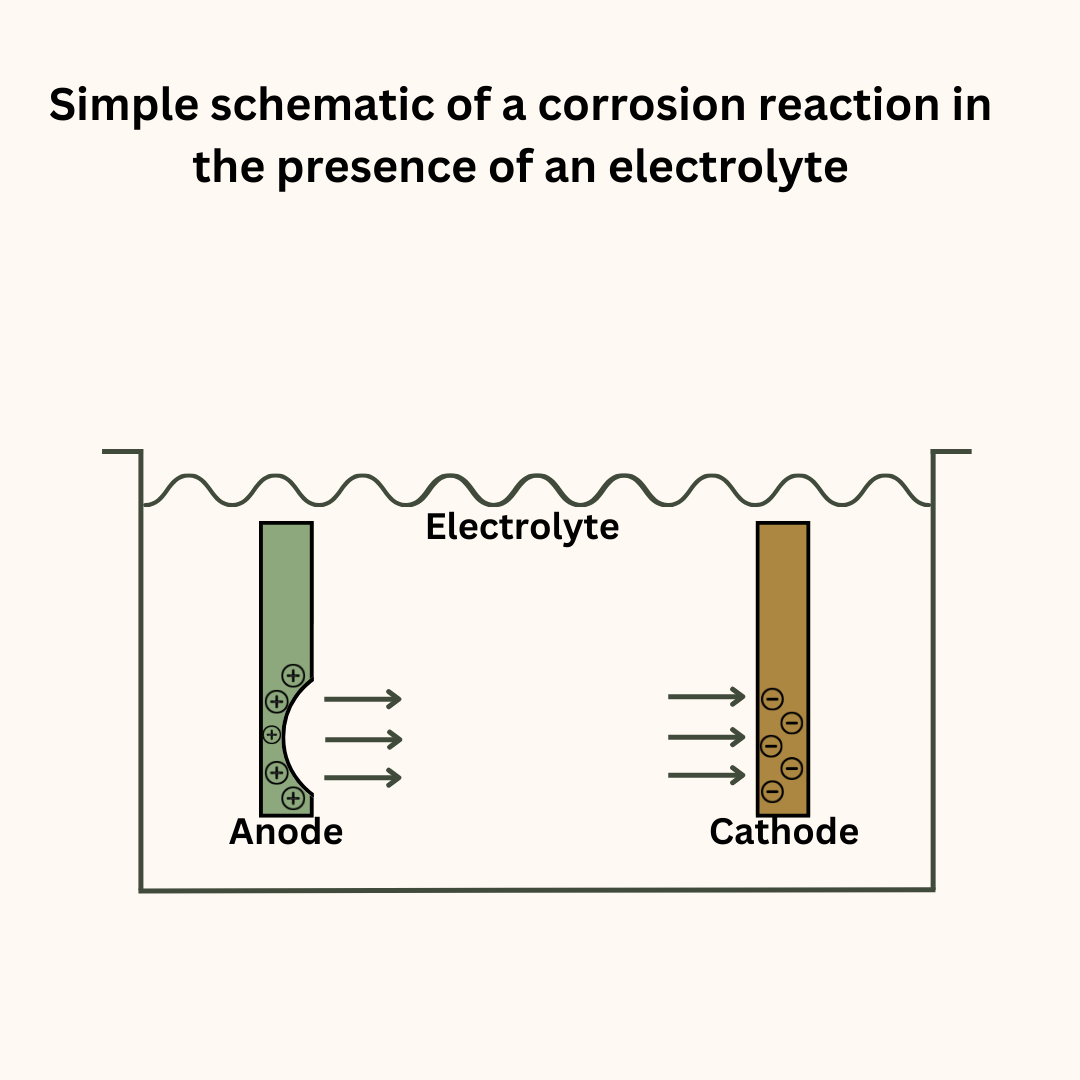
Metals that are susceptible to corrosion will decay or rust away without adequate protection from corrosion. Let us understand this phenomenon with the example of a large metallic structure immersed in seawater.
Do remember that the dissolved salts make the seawater an electrolyte. This makes an electrochemical reaction not only possible but also powerful. The following factors can set up a potential difference between the different parts of the structure:
-
Dissimilar metals
-
Structural and chemical non-uniformity
-
Weld quality and location
-
Seawater salinity, temperature and aeration
-
Varying paint quality and thickness in different areas
The more electropositive locations act as the anode and undergo corrosion. Thus, parts of the structure start to erode and thin down creating a dangerous situation. It also affects the structure’s mechanical properties such as tensile strength and fracture toughness.
Rectification of an underwater structure after corrosion is a difficult, costly and unreliable process. It is better to prevent it from happening. The same reasoning also applies to underground structures such as pipelines that are vulnerable to corrosion from the surrounding soil.
This is where cathodic protection helps.
The same concept as above can also be applied to other situations such as metal pipelines in ships and industries, metal tanks, and other metal structures exposed to the elements.
How does cathodic protection work?
The principle of cathodic protection is very simple. In any electrochemical reaction, it is the anode that undergoes corrosion. To control the corrosion, we must control this reaction.
If in our reaction, the metal we need to protect becomes the anode, it will invariably corrode. But we can make it act as the cathode. When that happens, instead of oxidation, the metal will undergo oxygen reduction and become safe from electrochemical corrosion.
One way to do this is to place a more electropositive element in the electrolyte. Electropositive elements are elements that are more willing to give up their electrons and undergo oxidation.
Thus, the new element becomes the anode and the metal to be protected becomes the cathode. The current direction changes and the corrosion is stopped.
A second method is to change the direction of the current by sending a reverse current through the metals. Let us look at each of these methods in detail.
Types of cathodic protection
The best way to prevent corrosion in metals is to prevent the formation of an electrochemical cell. This is possible through careful selection of the material and considerations during the design process.
But in many applications, an electrochemical cell formation is unavoidable. We have to use certain dissimilar metals because of their properties and they may develop contact through a liquid electrolyte.
In such cases, we can use the following cathodic protection systems to prevent corrosion due to an electrochemical attack.
-
Sacrificial anode cathodic protection
-
Impressed current cathodic protection (ICCP)
Sacrificial anode
In this method, sacrificial anodes are placed strategically throughout the system that is exposed to a corrosive compound. The chosen metal for the sacrificial anode is always more electropositive than the metal to be protected.

As the sacrificial anode is higher in the electrochemical series, the electrochemical attack occurs on the sacrificial anode instead of the system material.
Over time, the sacrificial anode gets eroded and must be replaced. Delays in replacement will lead to system corrosion until replacement.
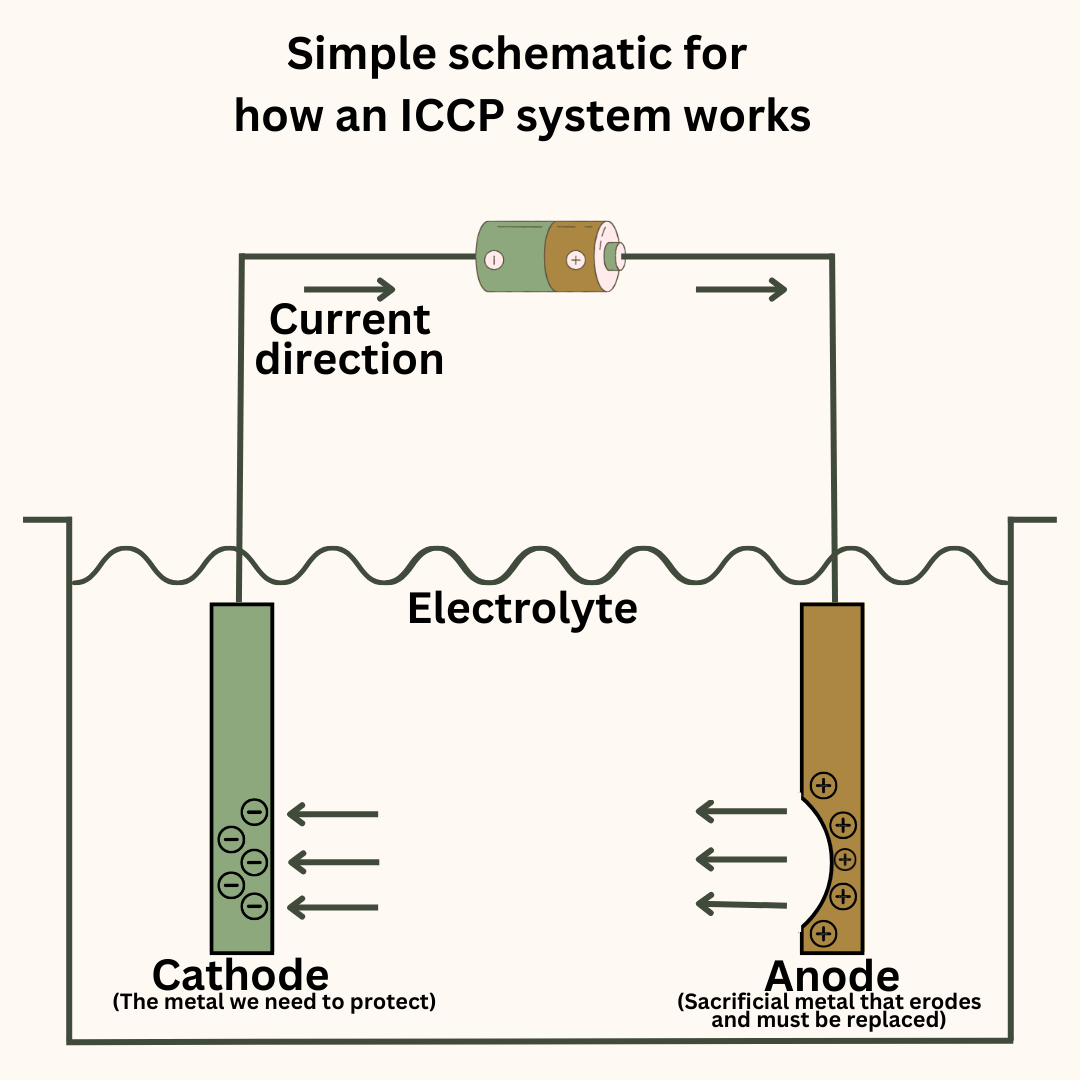
Impressed current cathodic protection (ICCP)
In this method, we force or impress a current in the opposite direction of the natural current direction to prevent corrosion of the metal we need to protect.
This method is popular for the protection of a ship’s hull but it also works to stop the corrosion of steel reinforcement in concrete structures.
As we saw earlier, a number of reasons cause the potential difference between the different parts of a metallic structure. The same list of reasons applies to a metal hull submerged in water.
The ICCP system has reference cells and anodes placed at suitable locations around the hull. The reference cells sense the electric potential at the different points of the hull and inform the ICCP control unit.
The control unit sends an appropriate amount of current to the anodes. This current leaves the anode, enters the seawater and through it, reaches the different parts of the hull.
What this does is essentially make the hull act as the cathode instead of the anode and there is no oxidation at the hull’s surface.
Uses of cathodic protection
Cathodic protection is suitable for a variety of applications. It is highly effective at eliminating or minimising the corrosion of vulnerable metallic structures. Some of the applications for cathodic protection are as follows:
-
Underground and underwater pipelines
-
Pipelines that carry corrosive compounds
-
Storage tanks
-
Condenser water boxes
-
Offshore platforms
-
Boat and ship hulls
-
Oil well casings
-
Steel reinforcements in concrete structures
-
Underwater structures such as locks, dams, and harbours
Conclusion
Cathodic protection is a very effective corrosion prevention method that has been in use for about 200 years since its first application by Sir Humphry Davy to protect British naval ships from corrosion in 1824.
He used iron anodes to protect copper-clad British ships. Over the years, many other methods have been discovered and applied such as corrosion inhibitors, protective coatings, alloying with certain metals and so on.
But cathodic protection still remains one of the most reliable and widely used corrosion protection methods.
FAQ
Why ICCP is switched off in port?
The ICCP system is stopped in harbours and fresh waters. In fresh waters, since the water becomes a less potent electrolyte, there is no need for the ICCP system. Whereas in harbours, the ICCP system will attempt to protect the harbour along with the ship’s hull and overload itself.
